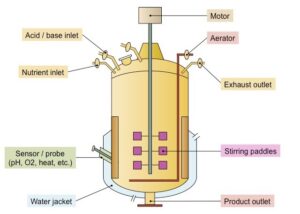
In the field of biotechnology and pharmaceuticals, bioreactors and fermenter play a crucial role in the large-scale production of biologically active compounds. Although these terms are often used interchangeably, they have distinct differences in terms of functionality, application, and design. Understanding these differences is essential for industries involved in microbial, enzymatic, and cell culture-based production.

What is Fermenter
A fermenter is a piece of equipment specifically designed to carry out fermentation processes, where microorganisms such as bacteria, yeasts or fungi transform organic substances into products such as alcohol, organic acids and gases. Fermenters are common in the food and beverage industry.
They provide an optimal environment for microbial growth by maintaining temperature, pH, oxygen levels, and nutrient supply. Common applications of fermenters include the production of antibiotics, alcoholic beverages, probiotics, enzymes, and organic acids. Depending on the process, fermenters can be aerobic or anaerobic, enabling industries to maximize yield and efficiency in biological manufacturing.
Key features of a fermenter
Fermenters are used especially in the production of fermented foods and beverages such as beer, wine, yoghurt and fermented soy products. In the pharmaceutical industry, they are used to produce antibiotics and other biotechnological products by microbial fermentation.
- Specific application: Designed primarily for fermentation processes.
- Control of conditions: Allows precise control of factors such as temperature, pH and oxygen, essential for fermentation.
- Materials of construction: Generally made of stainless steel to prevent contamination and facilitate cleaning.
- Process types: Handles both aerobic (with oxygen) and anaerobic (without oxygen) processes.
What is Bioreactor
A bioreactor ⇀ is a more versatile piece of equipment used for a variety of bioprocesses, not just fermentation. It is used in the production of biotechnological products such as proteins, vaccines, monoclonal antibodies and stem cells. Bioreactors can grow animal and plant cells, as well as microorganisms.
Bioreactors are widely used in pharmaceuticals, biotechnology, biofuel production, and wastewater treatment. They play a crucial role in vaccine manufacturing, monoclonal antibody production, enzyme synthesis, and tissue engineering. Unlike fermenters, which focus on microbial fermentation, bioreactors can support a broader range of biological processes, including cell culture and biopharmaceutical production, making them essential in modern industrial biotechnology.
Key features of a bioreactor
Bioreactors have different applications in the pharmaceutical ⇀ industry for the production of drugs, such as monoclonal antibodies and vaccines. They are also used in the production of biofuels, bioplastics, and in stem cell and gene therapy research.
- Versatility: Used for a wide range of processes, not just fermentation.
- Scalability: It is very varied, ranging from small volumes in laboratories to large volumes for industrial production.
- Advanced control: Includes precise monitoring and control systems to optimise culture conditions, such as dissolved oxygen, CO2, pH and temperature sensors.
- Diverse applications: Used in the pharmaceutical industry, biofuel production, stem cell research, and more.
| Feature | Bioreactor | Fermentor |
| Definition | A vessel used for biological reactions involving cells or microorganisms. | A vessel specifically designed for fermentation by microorganisms. |
| Scope | Used in pharmaceuticals, biofuels, tissue engineering, and wastewater treatment. | Primarily used in antibiotic, enzyme, and alcohol production. |
| Microorganism Type | Supports microbial, plant, and animal cell cultures. | Primarily used for microbial culture. |
| Oxygen Requirement | Used for both aerobic and anaerobic processes. | Typically used for anaerobic and aerobic fermentation. |
| Types of Products | Produces vaccines, monoclonal antibodies, recombinant proteins, and biofuels. | Produces antibiotics, organic acids, alcohols, and fermented foods. |
| Complexity | More complex in terms of operation and monitoring systems. | Less complex compared to bioreactors. |
| Design Variations | Stirred tank, airlift, packed-bed, and fluidized-bed types. | Batch, fed-batch, and continuous fermentors. |
Main differences between a fermenter and a bioreactor
- Application: The fermenter is more focused on specific fermentation processes, while the bioreactor is more versatile and used in a variety of biological processes.
- Design and construction: Although both can be made of similar materials, bioreactors tend to have more advanced and complex control systems.
- Versatility: Bioreactors offer greater flexibility in terms of the types of organisms and processes they can support.
- Process control: Bioreactors are equipped with advanced control and monitoring technologies, allowing for accurate, real-time management of culture conditions. Fermenters tend to be less complex in comparison.
- Scalability: Both can be scaled up for industrial production, but bioreactors have an advantage in the variety of scales and process types they can handle, from research to commercial production.
Uses of Bioreactors and Fermentors
Bioreactors and fermentors play vital roles in biotechnology, pharmaceuticals, and industrial microbiology. While both systems support microbial growth, their applications differ based on the biological process involved.
Uses of Bioreactors:
- Pharmaceutical Production: Used for manufacturing vaccines, monoclonal antibodies, and therapeutic proteins.
- Tissue Engineering: Supports cell culture for regenerative medicine and artificial organ development.
- Biofuel Production: Facilitates the growth of algae and microorganisms for biodiesel and ethanol production.
- Enzyme Manufacturing: Produces enzymes for industrial applications like food processing and detergent manufacturing.
- Wastewater Treatment: Helps in breaking down pollutants using microbial action in sewage treatment plants.
Uses of Fermentors:
- Antibiotic Production: Essential for the large-scale production of antibiotics like penicillin and streptomycin.
- Alcoholic Beverages: Used in the fermentation of beer, wine, and spirits.
- Probiotics and Organic Acids: Helps in the production of yogurt, vinegar, and citric acid.
- Enzyme Fermentation: Produces enzymes like amylase and protease for industrial use.
- Biogas Generation: Utilized for anaerobic digestion to produce methane-rich biogas.
Why Choose the Right System: Bioreactor vs. Fermentor?
Selecting the correct system—bioreactor or fermentor—is essential for optimizing production efficiency, maintaining product quality, and achieving industrial success. Both are critical in biotechnology and pharmaceutical applications, but their functions differ significantly.
1. Precision in Process Control
Bioreactors offer advanced control over temperature, pH, oxygen supply, and nutrient availability, making them suitable for delicate processes like tissue engineering, vaccine production, and enzyme synthesis. Fermentors, specifically designed for microbial fermentation, ensure controlled aerobic or anaerobic conditions for producing antibiotics, alcohol, and organic acids. Choosing the right system enhances yield, consistency, and product quality.
2. Cost-Effectiveness and Efficiency
Investing in the proper system reduces operational costs. Fermentors are ideal for companies focusing on fermentation-based products, offering a streamlined, cost-effective approach. Bioreactors, while more versatile, require additional control mechanisms, making them suitable for complex biological reactions. Choosing the correct equipment minimizes waste and optimizes resource utilization.
3. Industry-Specific Applications
- Bioreactors are widely used in pharmaceuticals, biofuels, tissue engineering, and wastewater treatment.
- Fermentors play a crucial role in food industries, probiotic production, and biogas generation.
A well-informed decision ensures alignment with industry needs, leading to greater productivity and sustainability.
4. Scalability for Future Growth
Whether for small-scale research or large-scale production, bioreactors and fermentors offer various scalability options. Understanding their differences enables businesses to expand operations efficiently and meet increasing demands without compromising quality.
Benefits of Understanding the Difference Between Bioreactor and Fermentor
In the field of biotechnology, pharmaceuticals, and industrial microbiology, bioreactors and fermentors play a crucial role in the controlled cultivation of microorganisms, cells, and biological substances. While both systems serve as vessels for biological processes, they have distinct applications. Understanding their differences and benefits can optimize production, enhance efficiency, and reduce costs for industries relying on bioprocessing.
1. Enhanced Process Control and Optimization
One of the biggest advantages of understanding the difference between a bioreactor and a fermentor is the ability to control and optimize production processes effectively.
- Bioreactors provide highly controlled environments for a variety of biological applications, including cell culture, enzyme production, and biofuel synthesis.
- Fermentors are specifically designed to facilitate microbial fermentation, making them ideal for antibiotic production, alcohol fermentation, and probiotic manufacturing.
By selecting the appropriate system, industries can ensure that oxygen levels, pH, temperature, and nutrient supply are optimized for their specific biological process, resulting in higher yields and better product quality.
2. Increased Efficiency and Cost Savings
Using the right system can significantly improve efficiency and reduce operational costs.
- Fermentors are optimized for microbial growth, meaning they require fewer modifications and specialized components, reducing initial investment and operating expenses for fermentation-based industries.
- Bioreactors, while more complex, allow for a broader range of applications, enabling multiple production processes within a single system. This flexibility can help industries cut costs by consolidating different biological processes into a single setup.
By choosing the correct equipment, industries can maximize production efficiency while minimizing resource waste, making their operations more cost-effective.
3. Improved Product Quality and Consistency
Whether producing pharmaceuticals, enzymes, or biofuels, maintaining high-quality and consistent product output is essential.
- Bioreactors provide precise control over environmental conditions, ensuring optimal cell growth and biological activity. This is crucial for vaccine production, monoclonal antibodies, and regenerative medicine, where consistency is key.
- Fermentors create controlled anaerobic or aerobic conditions for microbial fermentation, ensuring uniform antibiotic, enzyme, and alcohol production.
By using the right system, manufacturers can meet strict industry regulations, reduce batch variability, and enhance product reliability.
4. Broader Range of Applications
Understanding the differences between bioreactors and fermentors helps businesses choose the right technology for their specific needs.
- Bioreactors are versatile and used in pharmaceuticals, biopharmaceuticals, biofuels, wastewater treatment, and tissue engineering.
- Fermentors are commonly used in food, beverage, pharmaceutical, and biogas industries for large-scale fermentation processes.
This knowledge allows companies to expand their capabilities, explore new product lines, and increase market competitiveness.
5. Scalability and Future Growth
Choosing the appropriate system helps businesses scale their operations effectively.
- Fermentors are widely available in different sizes, from laboratory-scale to industrial-scale production, allowing companies to gradually expand their production capacity.
- Bioreactors offer advanced features such as automated control systems and real-time monitoring, making them suitable for large-scale production where precision and reproducibility are critical.
By selecting the right system, businesses can plan for future growth, meet increasing production demands, and maintain consistent quality as they expand.
Applications of Bioreactors and Fermentors
Bioreactors and fermentors are essential in biotechnology, pharmaceuticals, food production, and environmental applications. While both are used for cultivating microorganisms, cells, and biological substances, their applications vary depending on the process. Understanding these applications helps industries choose the right system for their specific needs.
Applications of Bioreactors
A bioreactor is a system designed to support biological reactions by providing a controlled environment. It is widely used in various industries, including pharmaceuticals, agriculture, and environmental science.
1. Pharmaceutical and Biopharmaceutical Industry
- Vaccine Production: Bioreactors are used to cultivate cells and viruses for vaccine manufacturing.
- Monoclonal Antibody Production: Essential in producing therapeutic antibodies for treating diseases like cancer and autoimmune disorders.
- Recombinant Protein Production: Used for synthesizing insulin, growth hormones, and other protein-based drugs.
2. Biofuel Production
- Ethanol and Biodiesel Production: Microorganisms like algae and yeast are cultivated to produce biofuels, reducing dependency on fossil fuels.
- Hydrogen and Methane Gas Generation: Bioreactors play a role in generating renewable energy through microbial action.
3. Enzyme Manufacturing
- Used for large-scale enzyme production, which is essential in industries like food processing, detergent manufacturing, and pharmaceuticals.
4. Wastewater Treatment
- Bioreactors help degrade pollutants and treat wastewater using microbial processes, making them crucial in environmental management.
5. Tissue Engineering and Regenerative Medicine
- Used in stem cell culture for regenerative medicine and artificial organ development.
- Supports 3D tissue growth for medical research and transplantation.
Applications of Fermentors
A fermentor is a type of bioreactor specifically designed for fermentation, where microorganisms convert sugars into desired products under controlled conditions.
1. Antibiotic Production
- Fermentors are extensively used for the mass production of antibiotics like penicillin, streptomycin, and erythromycin.
- They provide an optimized environment for microbial fermentation, ensuring high antibiotic yield.
2. Alcohol and Beverage Industry
- Used in the fermentation of beer, wine, and spirits, where yeast converts sugars into ethanol.
- Helps produce kombucha, kefir, and probiotic drinks that promote gut health.
3. Probiotic and Dairy Industry
- Fermentors facilitate the production of yogurt, cheese, and probiotic supplements by fermenting milk with beneficial bacteria.
4. Organic Acid Production
- Used in the manufacturing of citric acid, lactic acid, and acetic acid, which are essential in food preservation, cosmetics, and pharmaceuticals.
5. Biogas and Biofertilizer Production
- Anaerobic fermentors help convert organic waste into biogas (methane), a renewable energy source.
- Used in composting and biofertilizer production to enhance soil quality.
Conclusion:
Understanding the difference between bioreactors and fermentors is crucial for optimizing production in biotechnology, pharmaceuticals, and industrial microbiology. While both systems support microbial growth and biological processes, their applications vary significantly. Bioreactors offer a versatile platform for cell culture, enzyme production, vaccine development, and biofuel synthesis, whereas fermentors specialize in microbial fermentation for antibiotics, alcoholic beverages, probiotics, and organic acid production.
Choosing the right system enhances efficiency, product quality, and cost-effectiveness, ensuring businesses achieve their desired outcomes. Whether for large-scale pharmaceutical manufacturing or sustainable biofuel production, selecting the appropriate technology is key to success.